Research
Research in our group
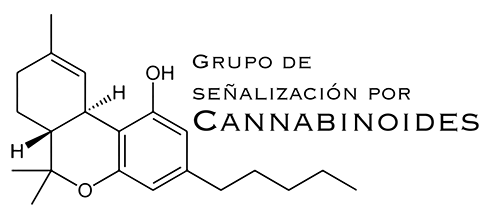
Marijuana (Cannabis sativa L.) and its derivatives have been used both medicinally and recreationally for at least fifty centuries. However, the chemical structure of their active components – the cannabinoids – was not elucidated until the early 1960s. Pharmacological studies conducted at that time led to the conclusion that, among all the cannabinoids present in the plant, one of them, D9-tetrahydrocannabinol (THC), was especially relevant owing to its high abundance and potency. Three decades later, the mechanism of THC action was unravelled. Thus, in the early 1990s it was found that THC acts on our body because it is similar to (and therefore mimics the effects of) a family of molecules produced by our organism that were so called endocannabinoids -anandamide and 2-arachidonoylglycerol being their main representatives. THC and endocannabinoids act by engaging specific proteins located on the surface of our cells and called cannabinoid receptors, two of which have been well characterized: CB1 y CB2. Nowadays it is known that these receptors, especially the former, are expressed preferentially in areas of the central nervous system that control processes such as motor behaviour, memory and learning, pain, appetite, nausea and vomiting, emotions and sensorial perception, which obviously explains that these processes are modulated by both the endocannabinoids generated at those locations and the THC that gains access to them owing to cannabis consumption. Cannabinoid receptors are also present at many other sites in our body, for example the peripheral terminals that innervate the skin and the digestive, circulatory and respiratory tracts, as well as in the immune system, the reproductive organs, the eye and the vascular endothelium. All these discoveries have contributed not only to an extraordinary expansion of the basic knowledge on how cannabinoids act in our body, but also to the renaissance of the study of their therapeutic properties, that constitutes a current topic of debate with ample scientific and clinical consequences.
In this context, by 1995 our research group started to study the molecular mechanisms by which endogenous and plant-derived cannabinoids act in the body. Specifically, our interest focused on unravelling whether these compounds impact cell generation, growth and survival, not only in the nervous system but also in other tissues. Understanding these processes is a pivotal issue for characterizing the aetiology and progression of neurodegenerative diseases (characterized by a loss of neural cells) and oncologic processes (characterized by an uncontrolled cell proliferation) and, therefore, for designing rational therapies for their treatment. Along these years we have observed for example that, upon activation of their specific receptors, cannabinoids modulate key cell signalling pathways, thereby eliciting effects such as progenitor cell generation, neuron and glial cell survival, and, on the contrary, apoptosis of brain, breast, skin and pancreatic cancer cells. These cellular events have a clear physiological relevance in laboratory animals, in which we have observed that cannabinoids, for example, control brain development, contribute to the regeneration and protection of nervous tissue upon damage, and inhibit the growth of malignant tumours. Overall, these studies support the notion that cannabinoid receptors are involved in the control of basic cell decisions and may thus constitute new pharmacological targets. However, further basic and preclinical research as well as thorough clinical trials are required to allow us to know whether cannabinoids could be used – aside from their palliative actions – as therapeutic agents in the treatment of neurodegeneration and cancer.
Neuroscience

Neurogenesis
The study of the signalling systems involved in neural stem cell proliferation, survival and differentiation is crucial to understand the development of the nervous system. Moreover, adult brain neurogenesis remains active in areas such as the hippocampus and the subventricular zone and may participate in cognitive functions and neurodegenerative disorders. Thus, our objectives include (i) to elucidate the role of the endocannabinoid system in cortical development and its potential regulatory action in pro-neurogenic and gliogenic genes; (ii) to investigate whether modulation of the endocannabinoid system in pathophysiological conditions such as brain excitotoxicity may attenuate some of the deleterious consequences of neurodegeneration.
For more information, please visit Ismael Galve-Roperh‘s site.

Neuroprotection
Understanding the processes of neural cell generation and survival is a pivotal issue for characterizing the aetiopathology of neurodegenerative diseases and, therefore, for designing rational therapies for their treatment. In this context, our group studies the neuroprotective role of the endocannabinoid system in various situations of brain damage, including Huntington’s disease (HD), a neurodegenerative hereditary disease produced by an expanded polyglutamine repeat in the amino-terminal portion of huntingtin and that nowadays has no cure. Our general goals include evaluating (i) whether alterations in the expression of endocannabinoid system elements are involved in the aetiopathology of neurodegenerative diseases, (ii) whether stimulation of the endocannabinoid system confers neuroprotection and therefore delays the onset and/or attenuates the progression of the neurodegenerative diseases, and (iii) which are the molecular and cellular mechanisms of the neuroprotective activity of the endocannabinoid system.
For more information, please visit Manuel Guzmán‘s site.
Oncology
Breast Cancer
Breast cancer accounts for approximately 30% of all diagnosed cancers. Although mortality associated to this disease is declining as a consequence of early detection, its incidence is permanently raising worldwide. Even though current therapies are at least partially effective in most cases, they are associated to undesirable side effects that dramatically reduce the patients’ quality of life. Moreover, certain cases do not respond to conventional medical treatments. Thus, it is obvious that new therapies are needed for the management of breast cancer. Considering the antitumoral potential of cannabinoids in animal models of other types of cancer, our aims are (i) to determine whether these compounds have similar effects on both in vitro and in vivo models of breast cancer, (ii) to characterize the mechanisms involved in these affects, with special emphasis on those that impact cell proliferation, and (iii) to determine whether the endocannabinoid system plays any role in oncogenesis and/or tumor progression. Since very recently, we are also interested in studying new cannabinoid receptors and their involvement in cannabinoid antitumoral action in breast and other cancers.
For more information, please visit Cristina Sánchez‘s site.

Brain Tumors
Glioblastoma multiforme (GBM), or grade IV astrocytoma, is the most frequent class of malignant primary brain tumor and one of the most aggressive forms of cancer. As a consequence, survival after diagnosis is normally just 6-12 months, a state of affairs that is due mainly to the high invasiveness and proliferation rate of GBM. In addition, GBM exhibits a high resistance to standard chemotherapy and radiotherapy. Accordingly, current standard therapeutic strategies for the treatment of GBM are only palliative, and include surgical resection and focal radiotherapy. Work performed in our laboratory during the last few years has shown that cannabinoid administration exert antitumoral actions in different models of tumor xenografts including gliomas. Based on these observations, current research in our laboratory is aimed at: (i) elucidating the molecular mechanisms responsible for these antitumoral actions and (ii) optimizing the potential therapeutic application of these agents. To this latter aim, we are investigating the molecular markers associated with the resistance to cannabinoid antitumoral action as well as exploring novel combinational therapies capable of enhancing the antineoplasic effects of these agents in gliomas.
For more information. please visit Guillermo Velasco‘s site.